44 year old male presents to ED with a medical history of hypertension and notes several vague complaints including numbness, fatigue, and slurred speech. Patient’s girlfriend notes that over the last several days the patient has been acting strangely, slurring his speech, appears confused, while periodically falling asleep.
Physical exam is grossly negative with the patient appearing to fall asleep several times during the exam. Of note, neurologic examination is found to be negative and subsequent workup includes mildly elevated liver enzymes. Lab values were otherwise largely benign with one glaring exception:
Alcohol intoxication is not a rare presentation by any means in the Emergency Department. It is on the much rarer occasion however that a patient will present with a toxic alcohol ingestion. These alcohols are similar in both name and clinical presentation making them perfect exam high yield material.
To review:
Anion Gap = Na – (Cl + HCO3)
Calculated Posm = (2 x plasma [Na]) + [glucose]/18 + [BUN]/2.8 + Ethanol/4.9
Osmolar Gap = Measured serum osmolality – Calculated osmolality
Osmolar gap > 10 is abnormal
By all means, use this as a guide to memorize right before exams, but moving forward these equations will serve as a guide to differentiate between different types of alcohol
Isopropyl Alcohol

Commonly known as rubbing alcohol, this is a household substance used as disinfectant, solvent or hand sanitizer1, which has also been noted to be used with the goal of intoxication and functions as a CNS inebriant and depressant. Although fatal overdoses are rare, the majority of symptoms are secondary to injury due to inebriant effects. The important laboratory distinction to be made is that this will cause an elevated osmolal gap without anion gap acidosis and a severe ketonemia1,4,5.
Toxicology
Isopropyl alcohol is metabolized to acetone leading to mild CNS depression. One of the important notes regarding the metabolism of isopropyl alcohol is that it metabolizes to a ketone, which is the cause of the ketonemia.
As alcohol dehydrogenase becomes saturated and isopropanol begins to build up the patient begins to experience CNS depression, respiratory depression, hypotension, and in rare conditions hemorrhagic gastritis1.
Although acetone contributes to the CNS depression, the primary driver of symptoms is isopropranolol. This means there is no role for fomepizole, as this would keep the reaction from progressing to it’s less toxic metabolite, acetone.
Diagnosis
Isopropyl alcohol and it’s product acetone will cause an osmolar gap without anion gap acidosis. This will be accompanied by ketosis noted to serum and urine.
Treatment
As discussed regarding the metabolism of isopropanol to the less toxic acetone, there is no role of fomepizole. Patients presenting with hypotension require IV crystalloid, and in rare cases of refractory hypotension, dialysis may be required. Recommendations for disposition include 6-8 hours of observation in the ED, with any patient having CNS depression requiring admission.
Methanol

This is an alcohol often used as a solvent and found in windshield washer fluid. The classic test presentation of methanol involves a patient drinking an unknown substance from the garage, followed by blindness. As with the other alcohols, history is paramount. The toxicity of methanol, anion gap and osmolar gap lend themselves to the zero order kinetics and formation of formic acid4,5.
Toxicity
As the levels of formic acid increase, this then leads to anion gap metabolic acidosis (acidosis from the formic acid, anion gap from change in chloride/Bicarb concentration). This is accompanied by the fact formic acid blocks oxidative phosphorylation and leads to lactic acidosis. Put simply, there is an anion gap metabolic acidosis
Formate causes damage to the retina and edema to the optic disc leading to the blindness seen with methanol poisoning. Edema of the optic disc can be seen on physical exam, and is thought to be a poor predictor of vision recovery moving forward.

Diagnosis
As discussed, this is an alcohol that metabolizes into an acid. Thus, similar to other alcohols there is an osmolar gap, but there is also an anion gap metabolic acidosis. History may point to blindness and physical exam will show edema of the optic disc. Due to the slow metabolism, symptoms may be prolonged.
Treatment4,5
- Loading dose of Fomepizole 15 mg/kg followed by 10 mg/kg for four doses. This treatment works due to the higher affinity of fomepizole (or ethanol at 800 mg/kg with continued dose of 100 mg/kg/h!) to alcohol dehydrogenase.
- Continue to monitor glucose levels as alcohol may induce hypoglycemia.
- End organ damage is an indication for dialysis which will remove methanol and ethylene glycol
- As methanol toxicity may lead to folate deficiency, administer 50 mg folinic acid
- 1-2 mEq/kg to maintain normal ph, and increased renal excretion of formic acid
- Consult toxicology
Ethylene glycol

Another alcohol found in the garage, ethylene glycol is found in anti-freeze, coolant, and de-icers. From an exam perspective, the important thing to keep in mind with ethylene glycol is the kidneys, osmol gap, and an anion gap. This will often be noted to fluoresce under Wood’s lamp, however this is neither sensitive nor specific.
Toxicity
The final product of calcium oxalate leads to two things, renal tubular necrosis and hypocalcemia.
There are three phases to ethylene glycol intoxication.
- First 12 hours: CNS effects without odor of ethanol
- 12-24 hours: Tachycardia, hypertension and tachypnea
- 24-72 hours: Renal effects such as flank pain, CVA tenderness, and acute tubular necrosis. Hypocalcemia may also be noted
Diagnosis
Early consultation with nephrology is indicated as dialysis may be necessary in the event of large intoxication or patients with anuria. These patients will have CNS depression, with non-specific symptoms such as hypertension, tachycardia, hyperventilation, prolonged QT and myositis. The main differentiators to keep in mind is the hypocalcemia, and the CVA tenderness, flank pain, calcium oxalate stones, oliguria or anuria which occur 24-72 hours after.
Academically, urine will have oxalate crystals, and fluorescence will be present. However this is non specific and should not be over interpreted.
Early in an ingestion, patients will have an Osmolar gap (refer to the formula!), but as ethylene glycol becomes calcium oxalate, it no longer is an alcohol and thus does not affect the osmolar gap. Patients will notably have an anion gap acidosis secondary to the metabolite calcium oxalate with labs indicating renal failure.
Treatment
- Early consultation to nephrology! Dialysis may be necessary with anuric acute tubular necrosis
- Similar to methanol, the goal is to competitively inhibit alcohol dehydrogenase with fomepizole.
- Monitor for hypoglycemia
- Administer pyridoxine 100 mg IV and thiamine 100 mg IV
- Sodium bicarbonate in the event of refractory/worsening acidosis.
- Disposition is 6 hours of observation, with continued CNS depression requiring admission.
Ethanol

Alcohol is a well known and easily recognized intoxication in the emergency department. Signs and symptoms are dependent on the level of intoxication2,5.
0.01-0.10 are associated with mild deficits in coordination, attention, euphoria
0.11-0.20 effect coordination, attention, ataxia, judgement, speech and mood
0.21-0.30 lack of coordination, confusion, nausea, vomiting
>0.31 results in stupor, loss of consciousness, coma and respiratory depression with the possibility of death
Toxicity
Unlike the previous alcohols, this can be measured by serum level. Similar to the rest of the alcohol intoxications, this will also have an osmolar gap Anion gap may also be present depending on level of intoxication.
Treatment
Fomepizole is not often used in alcohol intoxication as supportive care is largely the treatment for an acute ethanol intoxication. Along with this, disulfram has been noted to be used as an alcohol deterrent however has been noted to have low compliance due it’s effects of headache, vomiting, nausea, fatigue, and myalgias.
Case Conclusion
Returning to our case,
Keeping our formulas in mind:
Anion Gap = Na – (Cl + HCO3)
14 = 141 – (99 + 28)
Calculated Posm = (2 x plasma [Na]) + [glucose]/18 + [BUN]/2.8 + Ethanol/4.9
(2 x 141) + 114/18 + 7/2.8 + 500/4.9
282 + 6.3 + 2.5 + 102 = 392.8
Osmolar Gap = Measured serum osmolality – Calculated osmolality
419 – 392.8
26.2
we have a patient noted to have an osmolar gap, no anion gap, and CNS depression. There is no blindness and no renal dysfunction or calcium oxalate. This patient is acutely intoxicated by isopropyl alcohol!
It’s important to remember co-ingestions are common and a mixed picture could present itself with alcoholics. As a general rule of thumb however when suspecting a non-ethanol ingestion tools at your disposal include history, osmolar gap, anion gap, and physical exam.
Important clinical consideration
Keep in mind that clinical conditions are not as static as test questions. In real life, as the primary alcohol is metabolized, the ratio of osmolar gap decreases and anion gap metabolic acidosis increases4.
To put into example, as methanOL is metabolized, the osmolar gap decreases. Formic acid increases, thus increasing the metabolic acidosis. For exam purposes however, just take what you’ve learned here don’t extrapolate information.
Take home points
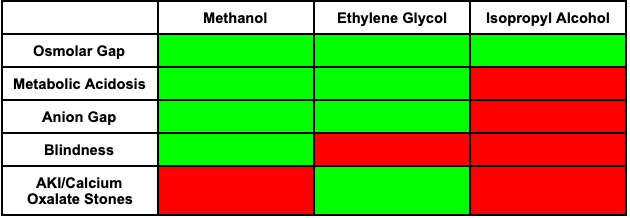
- History, Osmolar gap, Anion gap and physical exam will be your best friend for these cases and should take a step-wise fashion.
- Remember that as the alcohols metabolize, they have a decreasing effect on the osmolar equation ((2 x plasma [Na]) + [glucose]/18 + [BUN]/2.8 + Ethanol/4.9), and begin to have an increase in anion gap and acidosis.
- When a question stem mentions blindness, think Methanol!
- When a question notes calcium oxalate or hypocalcemia, think Ethylene Glycol and consult nephrology early!
Written by: Hakkam Zaghmout, MD
Peer reviewed and edited by: Stevley Koshy, DO
References
- Trullas JC, Aguilo S, Castro P, Nogue S. Life-threatening isopropyl alcohol intoxication: is hemodialysis really necessary? Vet Hum Toxicol. 2004 Oct;46(5):282-4. PMID: 15487656.
- Cowan, Ethan. “Ethanol Intoxication in Adults.” UpToDate, 2020, http://www.uptodate.com/contents/ethanol-intoxication-in-adults?search=ethanol&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1.
- Emmett, Michael. “Serum Osmolal Gap.” UpToDate, 2020, http://www.uptodate.com/contents/serum-osmolal-gap?search=methanol+poisoning&source=search_result&selectedTitle=3~39&usage_type=default&display_rank=3.
- Cohen, Jennifer. “185 Alcohols.” Tintinalli’s Emergency Medicine: a Comprehensive Study Guide, by David Cline et al., McGraw-Hill Education, 2020, pp. 1222–1232.
- The Ultimate Emergency Medicine Guide: the Only EM Book You Need to Succeed, by Sajid Khan, Createspace, 2018, pp. 275–277.


You must be logged in to post a comment.